Cos'è l'isomerismo EZ?
Risposta:
#E-Z# isomerism is a type of stereoisomerism that exists because of restricted rotation about double bonds.
Spiegazione:
In stereoisomers, the atoms are joined in the same order, but they have a different spatial arrangement.
In #E-Z# isomers you must have:
- restricted rotation, often involving a #"C=C"# doppio legame
- two different groups on one end of the bond and two different groups on the other end.
Un alchene come but-2-ene ha due gruppi diversi su ciascun carbonio alchenico.
Può esistere come #E-Z# isomeri che differiscono nelle posizioni dei sostituenti sugli atomi a doppio legame.

The substituents can be given "priorities", with atoms with higher atomic numbers given higher priorities (the Cahn-Ingold-Prelog rules).
If the highest priority groups for each carbon are on the same side of the molecule, we have the #Z# isomero.
If the highest priority groups for each carbon are on opposite sides of the molecule, we have the #E# isomero.
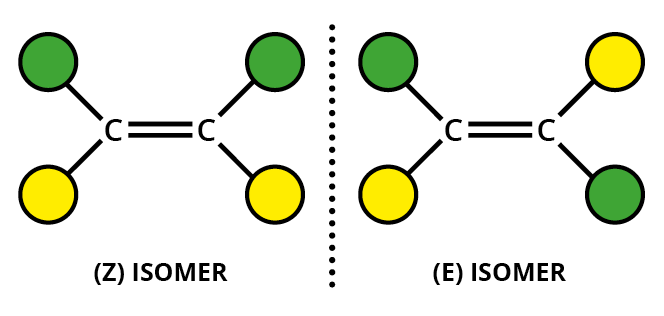
One way to remember the designations is to think of #Z# as Zame Zide (same side).
Dal #"CH"_3# ha una priorità più alta di #"H"#, it is pretty easy to determine that the butene isomers above are #E# e #Z#, Rispettivamente.
But what about the isomers of 2-methylbut-2-enoic acid (below)?

#"CH"_3# ha una priorità più alta di #"H"# e #"COOH"# ha una priorità più alta di #"CH"_3#.
Angelic acid has the higher priority groups on the same side, so it is the #Z# isomero.
Tiglic acid has the higher priority groups on opposite sides, so it is the #E# isomero.

