How many hybrid orbitals are present in the molecule PCl5?
Risposta:
There are no atomic hybrid orbitals in "PCl5.
Spiegazione:
A better question might be, "How many hybrid orbitals does a P atom use when it forms a molecule of PCl5?
VSEPR theory predicts that PCl5 should have a trigonal bipyramidal structure, which corresponds to an sp3d ibridazione.
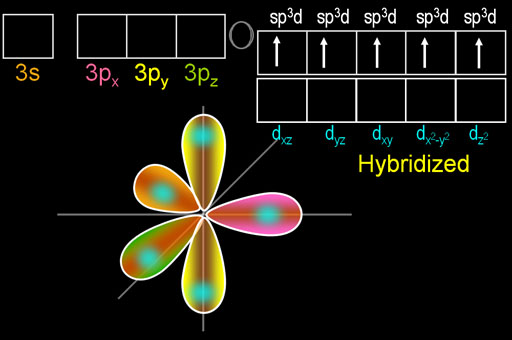
(Adapted from SlidePlayer)
The P atomo ha cinque orbitali ibridi.
We usually show the P-Cl bond as being formed by the overlap of a phosphorus sp3dorbital with a chlorine 3p orbitale.

(Adapted from SlidePlayer)
However, once a P−Cl bond has formed, the original atomic orbitals no longer exist.
They have become σ molecular orbitals.
Così, la PCl5 molecule has no hybridized atomic orbitals.

